Hello Ira!

Ira Sharma Sankhayan joins Yallow as Chief Compliance Officer.
Medical innovation with Cardiaccs
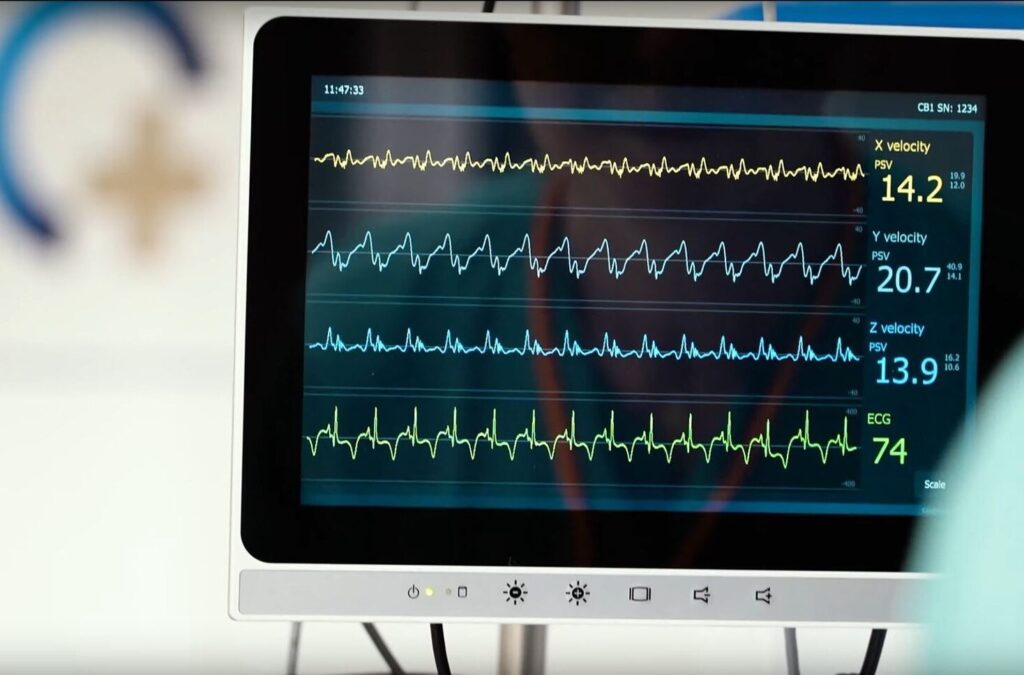
Yallow Life Science are happy to be part of the research and development team at Cardiaccs by assist them in engineering, software and electronic development.
Collaboration for innovation

Yallow Life Science and Observe Medical ASA enters into an agreement for collaboration.
Improving quality of life

Yallow and AssiTech have extended the agreement and will continue to work together also in 2021
Re-locating to Oslo Science Park

As of 1st of February 2021 Yallow will be at Oslo Science Park.
The team is growing

What a great start of this year. Another superstar joins the team and strengthen the product development team and capacity at Yallow.
Innovating for marginal gains with Zen Products Triathlon

We are happy to announce that we have a signed a partnership with Zen Products Triathlon.
Set out to save lifes!

We are very proud of being chosen as a partner to Asamedic and looking forward to a good collaboration.
Looking for QA/RA Manager

We are looking for a highly motivated, passionate and committed person who shares our simple, but important vision – innovate better life.
Yallow Training Session

Den 20. oktober holder Yallow en seminar på Forskningsparken med fokus på hvordan man effektivt kombinerer kreativ design tenking og de regulative kravene i design control.
